 Research Article
Research Article
The Relationship Between CD4+ Cell Count and Posterior Segment Findings in HIV-Positive Patients in Nigeria
Modupe O Arowolo1, Elizabeth A Awoyesuku2* and Adedayo O Adio2
1Saskatchewan Health Authority, Saskatchewan, Canada
2Department of Ophthalmology, University of Port Harcourt Teaching Hospital, Nigeria
Elizabeth A Awoyesuku, Department of Ophthalmology, University of Port Harcourt Teaching Hospital, Nigeria.
Received Date: December 19, 2019; Published Date: January 06, 2020
Abstract
Aim: To determine the relationship between CD4 leucocyte counts and posterior segment findings among HIV- positive patients in University of Port Harcourt Teaching Hospital, Nigeria.
Study design: A cross-sectional hospital-based study on adult HIV- positive patients attending the HIV clinic in University of Port Harcourt Teaching Hospital.
Methodology: Consecutive adult patients attending the Retroviral disease (RVD) clinic were included in the study. An informed consent was obtained and a structured interviewer administered questionnaire was used. Each patient had a comprehensive ophthalmic examination with dilated slit lamp bio microscopy to examine the fundus. Information on CD4 count was retrieved from the patient’s case notes. The data was analyzed using EPI-info version 7.0. Statistical methods such as the frequency and chi-square were used to test the significance of association. Level of significance was drawn at P<0.05.
Results: There was a female preponderance with a male to female ratio of 1:2. Fundal findings seen in HIV- positive patients were mostly retinal micro vasculopathy (4.6%) and occurred in patients with CD4 count below 200 cells/mm3.
Fundal findings seen in HIV- positive patients were mostly retinal micro vasculopathy (4.6%) and occurred in patients with CD4 count below 200 cells/mm3.
Keywords: Fundal findings; HIV positive; Tertiary hospital
Introduction
Human immune deficiency virus is an RNA virus. There are two types of HIV virus that has been described: HIV-1 and HIV-2. HIV-1 is common globally and more virulent while HIV2 is found commonly in the West African sub region. HIV primarily targets the CD4 lymphocytes because of the affinity of the virus for the CD4 surface marker. The CD4 T lymphocyte coordinates a number of important immunologic structures and loss of this results in progressive impairment of the immune response. The CD4 T lymphocyte count therefore serves as an important clinical and therapeutic guide in the management of HIV infection [1]. There are approximately 37.9 million people living with HIV at the end of 2018 [2].
It has long been recognized that the measurement of the blood level of HIV (that is the viral load) and the patients CD4 T cell count is fundamental to adequate management of infected patients [3,4]. Early after infection, both viral load and CD4 counts are relatively high, but as the disease progresses; the viral load often increases gradually while the CD4 level gradually decreases year by year. With the use of antiretroviral therapy, it tends to slow down the progression of the disease [2]. Good antiretroviral therapy and strict adherence are the only means currently available to ensure that patients control their viral infection, re-grow their immune system and improve their survival [5]. Combination therapy for HIV can reduce viral loads to undetectable levels [6]. The viral load and CD4 count are monitored from the patient’s first visit and then 4 - 6 monthly intervals. The CD4 count can be used in situations where viral load testing cannot be done. A patient with a failure to increase in CD4 count with the commencement of antiretroviral therapy and with new symptoms or new AIDS defining illness will call into question the adequacy of viral control.
The CD4 count has been used as a reliable predictor of ocular manifestations in HIV/AIDS [7,8]. The use of Highly Active Anti- Retroviral Therapy (HAART) has enabled the increase in CD4 count of these patients with a resultant decrease in ocular complications such as Cytomegalovirus (CMV) retinitis. Spontaneous resolution of CMV retinitis in patients with increased CD4 count has been reported [9]. Studies have shown that there is no benefit in commencing ART in asymptomatic patients with CD4 cell count > 350 cells/mm3 [10,11]. However, patients who have severe symptomatic though non-AIDS defining disease and those whose CD4 count is < 200 cells/mm3 must be offered ART [12-17]. There have been various ocular manifestations of HIV/AIDS described; some occur in an atypical and more severe form than what would be seen in a non-retroviral positive patient. The manifestations are influenced by the CD4 count and the viral load. With the advent of HAART, the occurrence and severity of some of the manifestations has reduced. In the earliest stage, non-specific ophthalmic involvement can occur but as the disease progresses, retinal micro vasculopathy occurs, later followed by opportunistic infections such as cytomegalovirus retinitis [18].
Posterior Segment Manifestations
This can be categorized into the following groups [18]
a) Opportunistic infections
b) Retinal vasculopathy
c) Unusual malignancies
d) Neuro-ophthalmic abnormalities
Opportunistic infections
Cytomegalovirus retinitis (CMV): This is a common opportunistic infection of the posterior segment in HIV/AIDS patients and can occur in up to 40-50% of Patients prior to HAART [19]. Where treatment with HAART is available; the incidence of new CMV disease has fallen by 80% [20]. CMV has a worldwide distribution and transmission may occur at birth and during early childhood. Symptomatic CMV in HIV infected patients usually only becomes a problem when CD4 cell counts are extremely low, (<50 to 100 cells/mm3). CMV retinitis is a necrotizing form of retinitis and can be in an indolent or fulminant form. Patients usually report floaters, flashing lights, visual field loss or blurred vision.
Retinal vasculopathy
Micro vasculopathy is the most common ocular manifestation of AIDS seen in 40-60% of HIV-positive patients. Clinically seen as cotton wool spots, very few hemorrhages, mild vitritis. Its presence is inversely proportional to CD4 count level. Large vessel disease may also present including central and branch retinal vein or artery occlusions [21,22]. Acute Retinal necrosis is a fulminant retinal vaso-occlusive necrotizing retinitis which tends to occur at CD4 counts above 60/μl and associated varicella zoster virus [23].
Unusual malignancies
Lymphoma tends to involve the vitreous and retina and may masquerade as uveitis. It can present as chronic anterior uveitis, intermediate uveitis and in the posterior segment as multifocal, large yellowish, sub-RPE infiltrates which can coalesce in a ring form pathognomonic of intraocular lymphoma [24]. Its presentation is usually bilateral but may be asymmetrical. Lymphoma has been associated with AIDS [25]. Epstein Barr virus was associated with a case of intra ocular lymphoma reported by Mittra, et al. [26].
Neuro-ophthalmological manifestations
These are seen in 10-15% of HIV patients and include papilloedema, cranial nerve palsies, ocular motility disorders and visual field defects [27].
Material and Methods
Consecutive adult patients attending the Retroviral disease (RVD) clinic were included in the study. An informed consent was obtained, and a structured interviewer administered questionnaire was used. Each patient had a comprehensive ophthalmic examination with dilated slit lamp bio-microscopy to examine the fundus. Other information such as CD4 count and HIV serotype were retrieved from the patient’s case notes. The data was analyzed using EPI-info version 7.0. Statistical methods such as the frequency and chi-square were used to test the significance of association. Level of significance was drawn at P<0.05.
Results
A total of 153 patients had ocular manifestations of HIV/AIDS out of 411 patients studied. The demographic characteristics are as shown in (Table 1,2).
Most posterior segment manifestations occurred in patients with CD4 count below 200 cells/mm3. (Table 3)
Optic atrophy was the only neuro ophthalmic manifestation seen with 50% occurring between CD4 count of 101-200 cells / mm3 and 50% occurring in CD4 count above 500 cells /mm3. Most patients (99%) did not have any neuro-ophthalmic manifestations (Table 4).
Table 1: Demographic characteristics of study population.

Table 2: Relationship between CD4 level and posterior segment presentation.
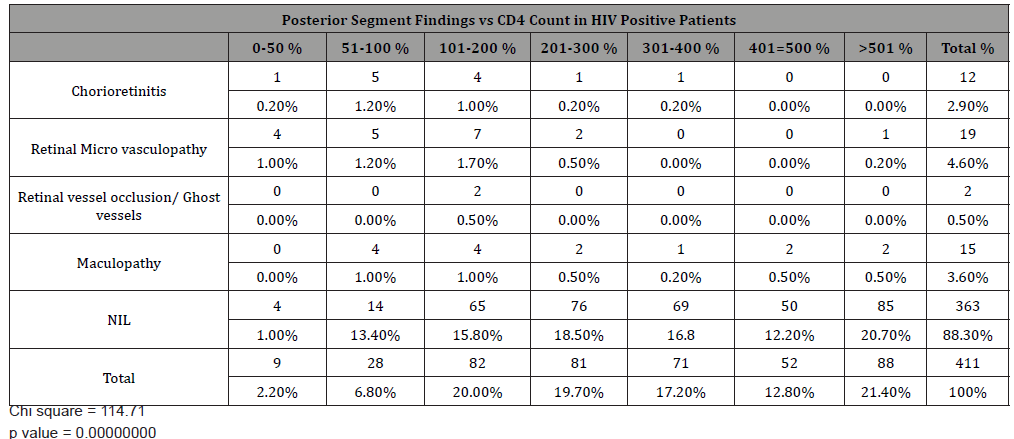
Table 3: Neuro -Ophthalmic manifestations of 411 patients studied versus CD4 count.
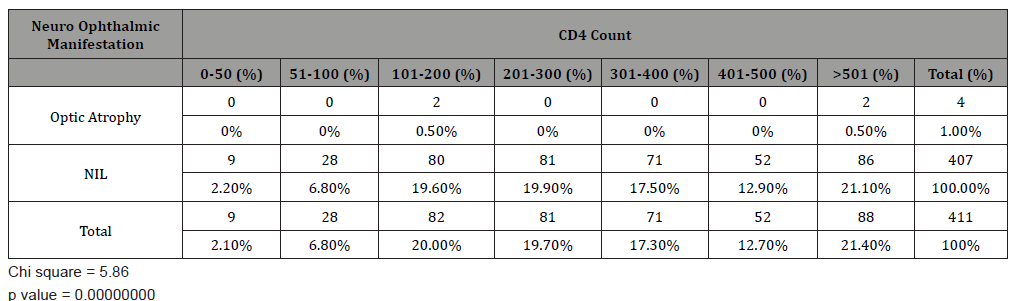
Table 4: Relationship between ocular manifestations and CD4count among the 411 HIV positive patients.
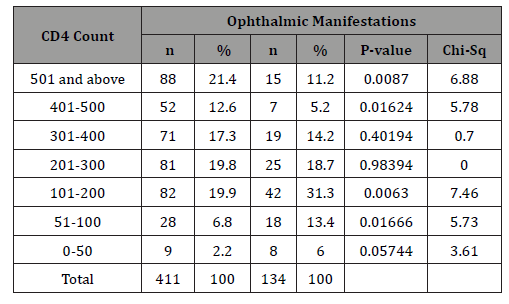
There was a significant association between the ocular manifestations and most CD4 count ranges.
Discussion
The demographic characteristics of our study population showed a female preponderance with most patients in their 3rd which agrees with most studies [28], Kumar P [29] however reported an all-male population in the 4th decade of life. HIV microangiopathy was the commonest posterior segment abnormality with average CD4 count above 200 cells/μL. Majority of ocular lesions occurred with CD4 counts between 100-200cells/μl. Our study revealed 71% cases with CD4 count >200cells/mm 3 and 2.1% had CD4 count < 50 cells/mm3. The pattern of the CD4 count was similar to that reported by Onunkwor [30] and Odeyemi [31]. Low CD4 count is a predictor for ocular manifestations with HIV microangiopathy being the commonest posterior segment manifestation and acute retinal necrosis presenting in patients with counts less than 100 cells/μl [32]. Bekele, et al. [33] found a statistically significant relationship between ocular manifestations between ocular manifestation CD4 counts. HIV microangiopathy were common in patients with CD4 less than 200 μL. Retinal micro vasculopathy was highest in frequency (4.6%). The exact pathogenesis is not clear but researches have shown that haemo dynamic abnormalities may be implicated. The commonest posterior segment manifestation in HIV in Cameroon was CMV retinitis [34]. A study by Banker [18] reported retinal micro vasculopathy and CMV retinitis as the most common manifestations.
Ocular toxoplasmosis is a frequent cause of infectious retinitis in our environment even in immunocompetent individuals. Atypical lesions can occur in HIV/AIDS patients which may be characterized by multifocal or extensive confluent areas of retinitis. Ocular toxoplasmosis was detected in 2.9% of the study population with chorioretinitis/chorio retinal scar. Some cases of ocular toxoplasmosis also presented with maculopathy. Other cases of maculopathy seen presented with cystoid changes around the macula, epiretinal membrane and pigmentary macula changes. These may have resulted from immune recovery uveitis. CMV retinitis has been reported as the leading ocular manifestation in many countries outside Africa even in the HAART era, [35]. The presentation in African region has not been reported as high. This may be due to early mortality, difference in race, HIV subtype or co-morbidity. There was no case of CMV retinitis in this study. The overall prevalence in Africa in patients with AIDS varied from 0-8.5%93-95.
Most posterior segment manifestations such as chorioretinitis, retinal vasculopathy, retinal vessel occlusion and maculopathy occurred in patients with CD4 < 200cell/mm3. This was similarly reported by Ndoye, et al. [35] There was a significant association between ocular manifestations and most CD4 count ranges. Complications of ocular manifestations may result from inflammation, nerve damage or tissue scarring [26,36]. In a study by Azonobi [38] 80% of patients with visual impairment had CD4 counts of 300cells/μl or less while Retrobulbar optic neuritis was the commonest cause of blindness.
Limitation of the Study
There was no indication of the HIV subtype in most patients laboratory result, thus it was not possible to determine the proportion of those with HIV 1 and 2.
Conclusion
Fundal findings seen in HIV-positive patients were mostly retinal micro vasculopathy (4.6%) and occurred in patients with CD4 count below 200 cells/mm3.
Acknowledgement
None.
Conflicts of Interest
No conflict of interest.
References
- The Science of HIV and AIDS- OVERVIEW.
- World Health Organization (WHO) HIV/AIDS Key facts.
- Pantaleo G, Graziosi C, Fauci AS (1993) The Immunopathogenesis of Human immunodeficiency virus infection. N Engl J Med 328(5): 327-335.
- Saag MS1, Holodniy M, Kuritzkes DR, O Brien WA, Coombs R, et al. (1996) HIV viral load markers in clinical practice. Nat Med 2(6): 625-629.
- Detels R, Tarwater P, Phair JP, Margolik J, Riddler SA, et al. (2001) Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS 15(3): 347-355.
- Siliciano R (1999) HARRT for life. Hopkins HIV Rep 11(4): 2.
- Biswas J, Kumaraswamy N, Solomon S (1999) Ophthalmic manifestations of Human immunodeficiency virus in India. Indian J Ophthalmol 47: 87-93.
- National HIV surveillance (2003) National AIDS/STD control programme: Research monitoring and evaluation unit, Ministry of Health.
- Hung Fan (2005) AIDS: Science and Society. (3rd edn) Jones and Barltelt (publishers), USA, pp. 45.
- Sterling TR, Chaisson RE, Moore RD (2003) Initiation of Highly Active Antiretroviral Therapy at CD4 & lymphocyte counts of >350 cells/mm3: disease progression, treatment durability, and drug toxicity. Clin Infect Dis 36(6): 812-815.
- Lepri AC, Phillips AN, Monforte AA, Castelli F, Antinori A, et al. (2001) When to start highly active antiretroviral therapy in chronically HIV- infected patients: evidence from the ICONA study. AIDS 15(8): 983-990.
- HIV/AIDS treatment Information Service (2002) Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents.
- World Health Organisation (WHO) (2002) Scaling up antiretroviral therapy in resource-limited settings. Guidelines for a public health approach. IAPAC Mon 8(6): 168-175.
- The British HIV Association (BHIVA) (2001) Guidelines on the use of antiretroviral therapy.
- Gizzard B, Murphy RL (2003) European guidelines for the management and treatment of HIV-infected adults in Europe. AIDS 17: 1-26.
- Adults Guidelines Committee (2002) Southern African HIV Clinicians society. Antiretroviral therapy in adults. Southern African J HIV Medicine 8: 22-29.
- Simelela NP (2004) National Antiretroviral treatment Guidelines. National Department of Health, South Africa p. 3.
- Banker AS (2008) Posterior segment manifestations of human immunodeficiency virus/Acquired immune deficiency Syndrome. Indian J Ophthalmol 56(5): 377-383.
- Jabs DA (1995) Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 93: 623-683.
- Jacobson MA, Stanley H, Holtzer C, Margolis TP, Cunningham ET (2000) Natural history and outcome of new AIDS- related cytomegalovirus retinitis diagnosed in the era of Highly Active Antiretroviral therapy. Clin Infect Dis 30(1): 231-233.
- Friedman SM, Margo CE (1995) Bilateral central retinal vein occlusions in a patient with acquired immunodeficiency syndrome: Clinicopathologic correlation. Arch Ophthalmol 113(9): 1184-1188.
- Park KL, Marx JL, Lopez PF, Rao NA (1997) Noninfectious branch retinal vein occlusion in HIV-positive patients. Retina 17(2): 162-164.
- Ocular Manifestations of HIV infection by Copeland RA and Phillpotts BA.
- Ormerod LD, Puklin JE (1997) AIDS-associated Intraocular lymphoma causing primary retinal vasculitis. Ocul Immunol Inflamm 5(4): 271-278.
- Schanzer MC, Font RL, Malley RE (1991) Primary ocular malignant lymphoma associated with the acquired immune deficiency Syndrome. Ophthalmology 98(1): 88-91.
- Mittra RA, Pulido JS, Hanson GA, Kajdacsy Balla A, Brammitt CF (1999) Primary ocular Epstein-Barr virus associated non-Hodgkin’s lymphoma in a patient with AIDS: a clinicopathologic report. Retina 19(1): 45-50.
- Feroze KB, Wang J (2019) Ocular Manifestations of HIV. Stat Pearls Publishing LLC.
- Nigeria Overview: UNAIDS.
- Kumar P, Vats DP, Mishra S, Makkar A, Banarji A, et al. (2011) CD4 counts: a strong indicator of retinal and ocular lesions in HIV disease. Med J Armed Forces India 67(4): 354-357.
- Onunkwor CI (2007) Ophthalmic manifestation in HIV/AIDS patients receiving antiretroviral therapy.
- Odeyemi B (2009) Ocular disorders in HIV/AIDS patients attending PEPFAR clinic at the Lagos University Teaching Hospital, Nigeria.
- Gogri PY, Misra SL, Kothari RN, Bhandari AJ, Gidwani HV (2014) Ophthalmic Manifestation of HIV patients in a rural area of Western Maharashtra, India. Int Sch Res Notices 2014: 347638.
- Bekele S, Gelaw Y, Tessema F (2013) Ocular manifestation of HIV/AIDS and correlation with CD4+ cells count among adult HIV/AIDS patients in Jimma town, Ethiopia: a cross sectional study. BMC Ophthalmol 13: 20.
- Ebana MC, Ellong A, Bella AC, Lumah A, Achu Joko H (2007) Ocular complications of HIV/AIDS in Cameroon: Is there any correlation with the level of CD4 lymphocyte count? Bull Soc Belge Ophthalmol 305: 7-12.
- Ndoye NB, Sow PS, Ba EA, Ndiaye MR, Wade A, et al. (1993) Ocular manifestations of AIDS in Dakar. Dakar Med 38(1): 97-100.
- Azonobi IR, Tebepah T (2013) Visual Status and causes of low vision and blindness among HIV/AIDS patients in Yenagoa, Bayelsa State, Nigeria. J AIDS Clin Res 4: 206.
-
Elizabeth A Awoyesuku, Modupe O Arowolo, Adedayo O Adio. The Relationship Between CD4+ Cell Count and Posterior Segment Findings in HIV-Positive Patients in Nigeria. W J Opthalmol & Vision Res. 2(5): 2020. WJOVR.MS.ID.000552.
-
CD4+ Cell Count, Posterior segment, HIV-Positive Patients, Lymphocyte, Therapeutic guide, Human, Immune, Deficiency, Antiretroviral therapy, Cytomegalovirus, Vasculopathy, Fulminant form
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






